Studies highlight critical role of BORC protein complex in nervous system development
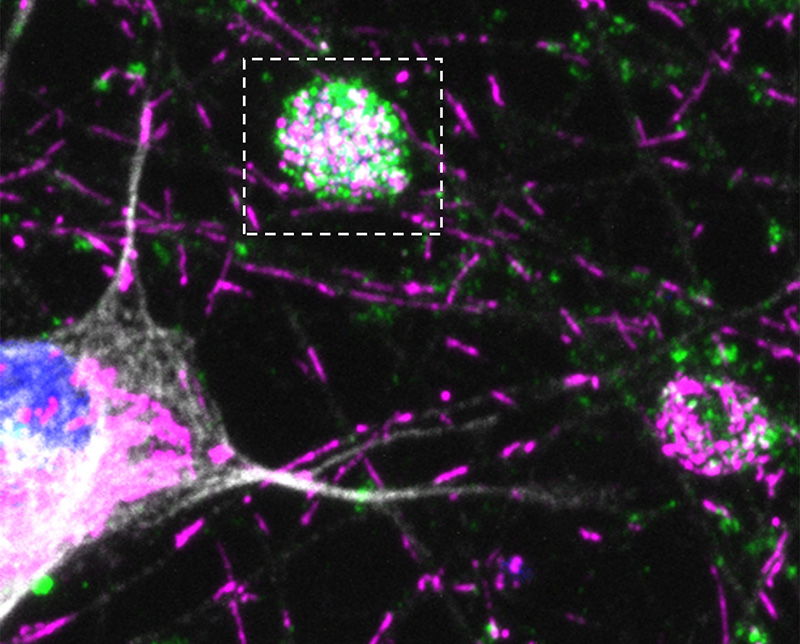
Credit: Bonifacino Lab, NICHD
Genetic variants that disable the protein BORCS8 cause a severe neurodegenerative disorder that begins early in life, according to research by NIH scientists and colleagues. The findings, published in Brain, highlight the importance of BORCS8, a component of the protein complex BORC, in nervous system development and function. Additional work by the NIH researchers, appearing in Nature Neuroscience, unravels the role of BORC in neurons and explains how defects in BORC function may contribute to neurodegeneration.
Background
The BORCS8 gene provides instructions for the BORCS8 protein, which is one of eight proteins comprising BLOC-one-related complex (BORC). BORC regulates the positioning of lysosomes, the structures within cells that break down unneeded or worn-out cell parts. The ability of lysosomes to move within cells is critical for many cellular functions, including those important for immunity, cell repair, cell migration, and cancer progression. Problems with lysosomes also can contribute to neurological diseases. Although BORC is present in all human cells, BORC deficiency primarily affects the central nervous system.
Results
Researchers from NIH’s Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and colleagues identified four BORCS8 variants in five children with a severe neurodegenerative disorder that began during infancy. These children, from three unrelated families, experienced developmental delays, severe-to-profound intellectual disability, decreased muscle tone, muscle wasting, and other signs of progressive neurodegeneration.
Experiments in cell culture established that these BORCS8 variants led to a partial or complete loss of BORC function, reducing or eliminating the cell’s ability to distribute lysosomes appropriately. Deleting the analogous borcs8 gene in zebrafish led to lower brain and eye size, neuromuscular anomalies, and impaired movement. Together, these findings indicate that the BORCS8 variants identified in the children are responsible for their disease.
A subsequent study shed light onto the mechanism by which BORC deficiency leads to neurodegeneration. NICHD scientists found that halting lysosome transport in neurons by depleting BORC prevented trafficking of certain mRNAs into the axon, a part of the neuron that enables it to communicate with other nerve cells. Many of these mRNAs encode proteins important for the function of mitochondria—the cell’s energy producers. Decreased production of these mitochondrial proteins in the axon led to defects in the mitochondria and eventually to degeneration of the axon.
Significance
The findings reveal that BORCS8 is critical for the development and function of the central nervous system. They also help explain how defects or deficiencies in BORC may contribute to neurodegenerative diseases.
References
De Pace R, Maroofian R, Paimboeuf A, et al. Biallelic BORCS8 variants cause an infantile-onset neurodegenerative disorder with altered lysosome dynamics. Brain DOI: 10.1093/brain/awad427 (2024)
De Pace R, Ghosh S, et al. Messenger RNA transport on lysosomal vesicles maintains axonal mitochondrial homeostasis and prevents axonal degeneration. Nature Neuroscience DOI: 10.1038/s41593-024-01619-1 (2024)

 BACK TO TOP
BACK TO TOP